YO Home Sperm - 3 Test Kit
A private, easy way to check sperm quality at home with no lab visit or mail-in needed. With over 97% accuracy, YO measures Sperm Concentration, Motility, Progressive Motility, Motile Sperm Concentration, and Progressive Motile Sperm Concentration and even records a live video of your sample. Doctor-recommended, FDA-cleared, WHO 6th Edition Compliant, and shipped discreetly.

Confidence Begins with Clarity
Private, stress-free, and accurate - take charge of your Fertility Journey with YO!
Easy & Stress-Free Semen Analysis
Testing him has never been simpler. Quick and Easy with no Lab Visits or Mail-In required.
Accurate Results You Can Trust
FDA-cleared and 97% accurate, YO provides reliable insights to support your reproductive journey.
Clinical Grade Results in Real-Time
Get accurate WHO 6th Edition results in just 20 minutes from the convenience of your smartphone.
Private and Secure Testing on Your Terms
Discreet testing in the comfort of home, with results encrypted and stored securely in the YO app.
Why YO vs. Other Options?
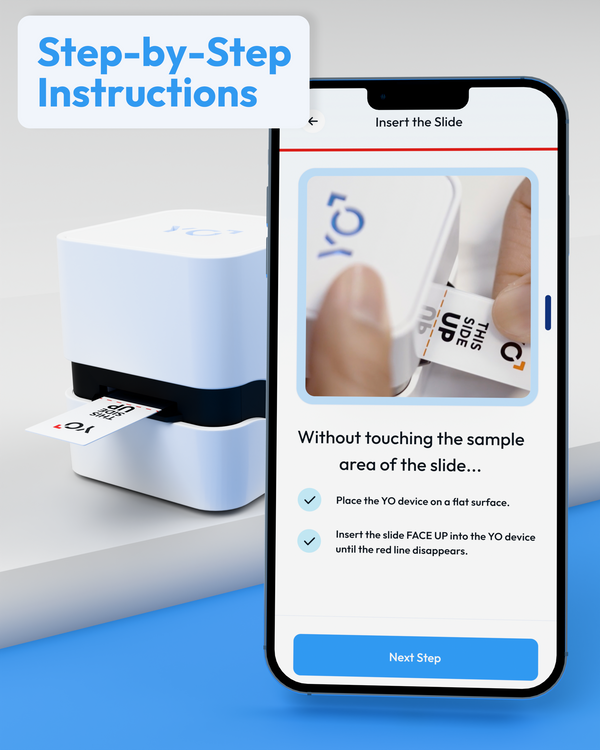
Simple Steps to Fertility Insights
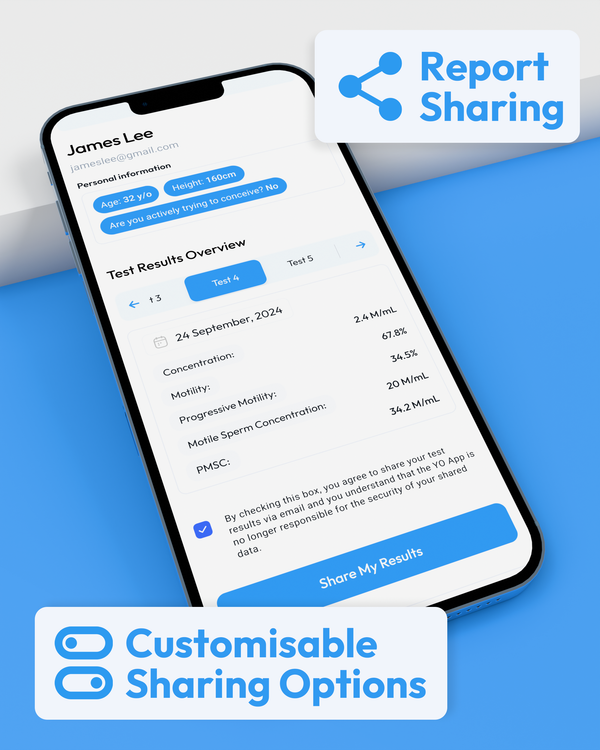
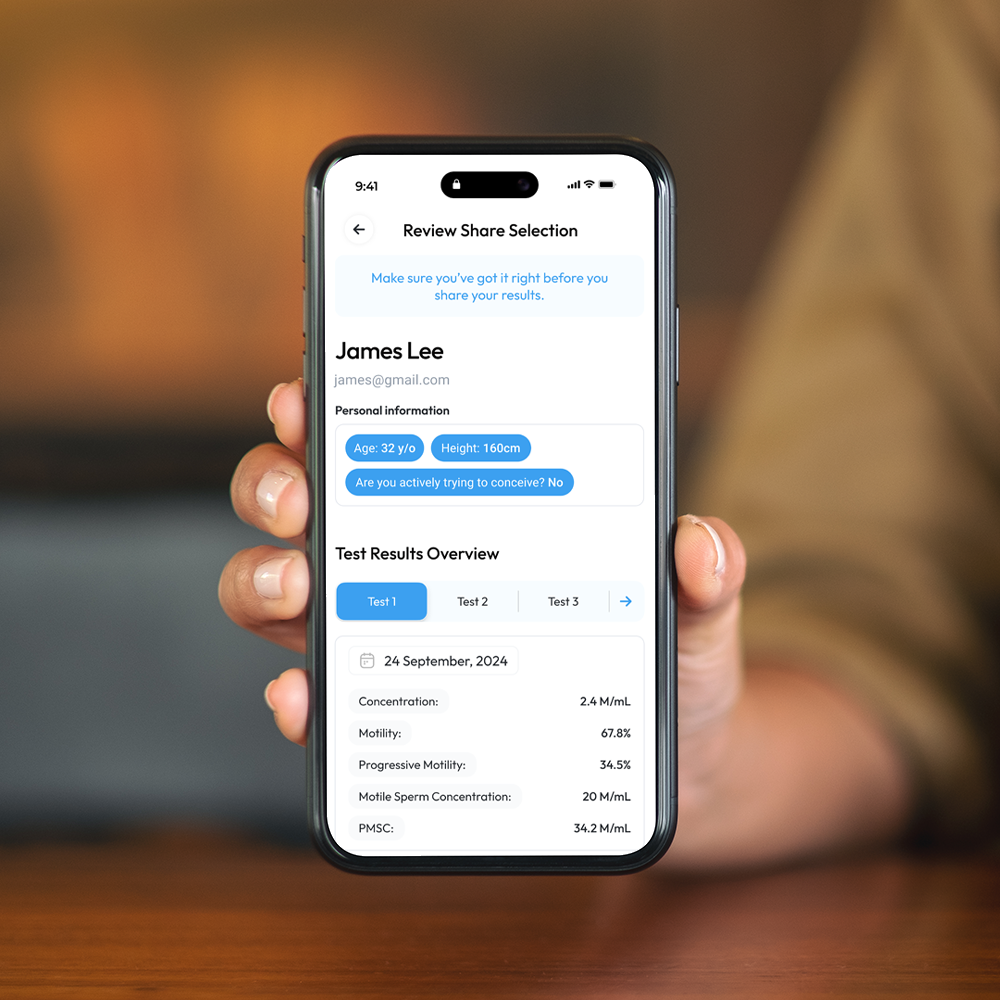
Comprehensive Results at Your Fingertips
WHO 6th Edition Semen Analysis Results in the Palm of your Hand.
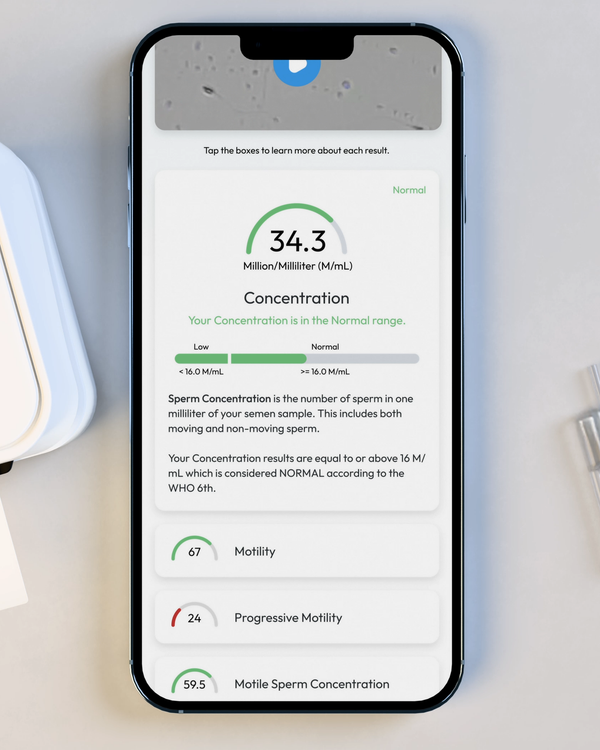
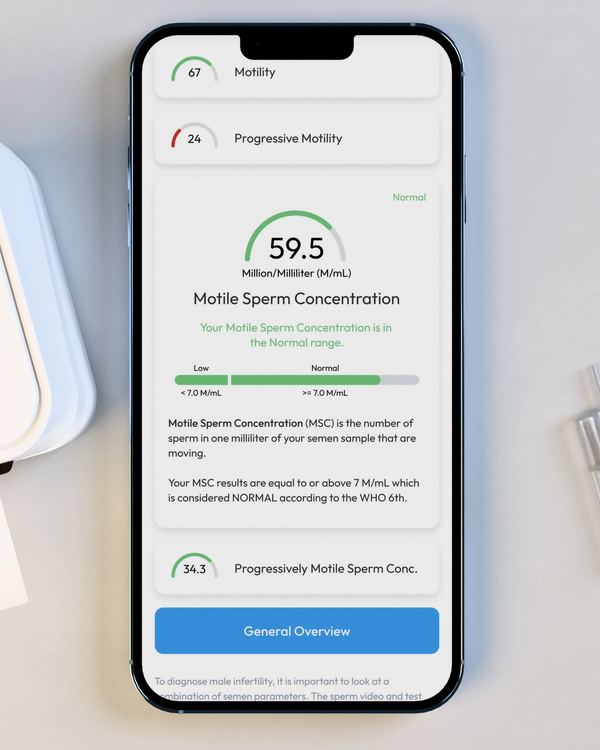
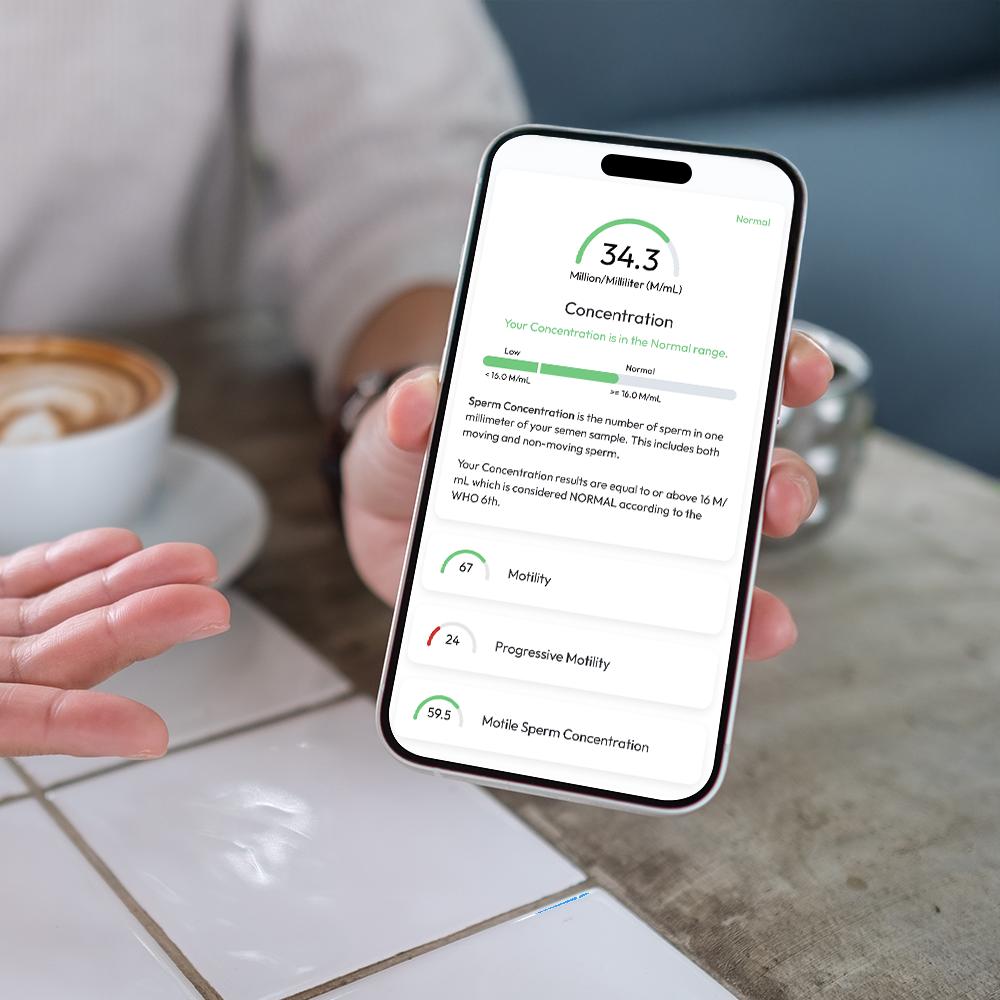
Sperm Concentration, M/mL
YO analyzes and reports the number of sperm cells in your semen sample (per ML.) This is sometimes called "Sperm Count."
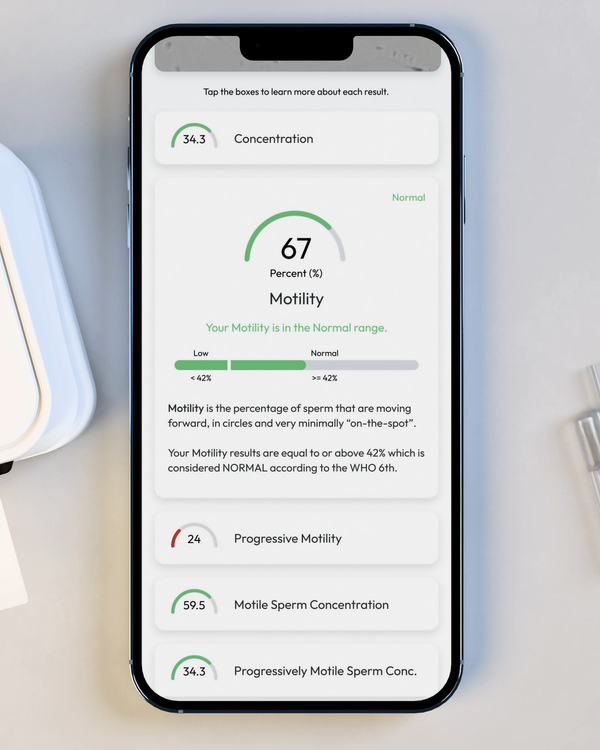
Sperm Motility, %
Motility is the percentage of your sperm cells that are alive and moving. This includes any type of active motion.
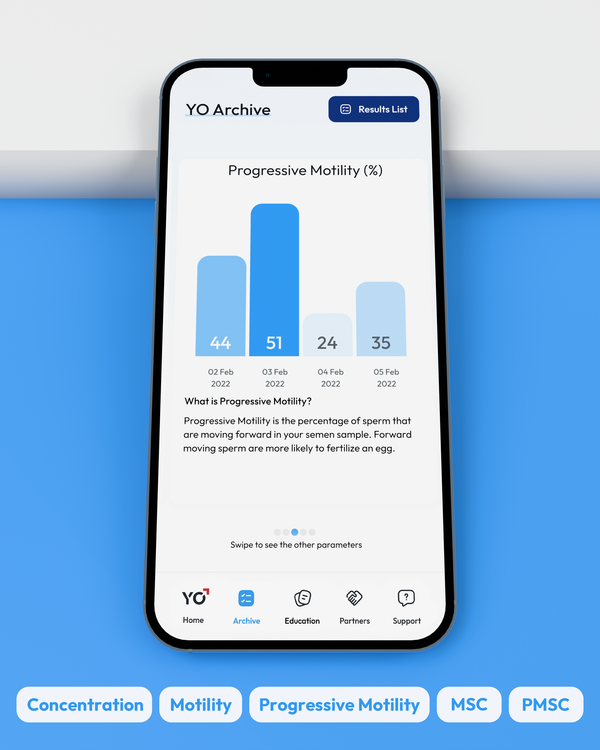
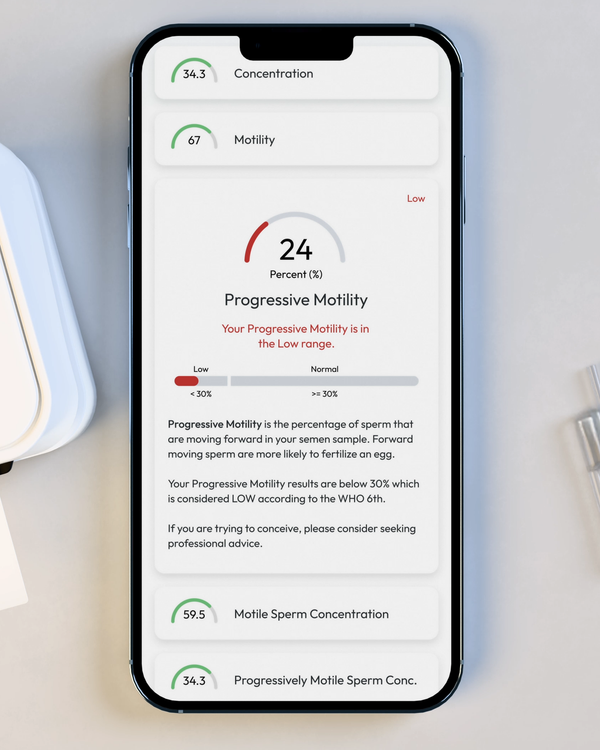
Progressive Motility, %
Progressive Motility is the percentage of sperm cells that are making forward progress, not just moving in place.
Motile Sperm Concentration, M/mL
Sometimes called "MSC", Motile Sperm Concentration is the number of Motile Sperm in your sample, per ML.
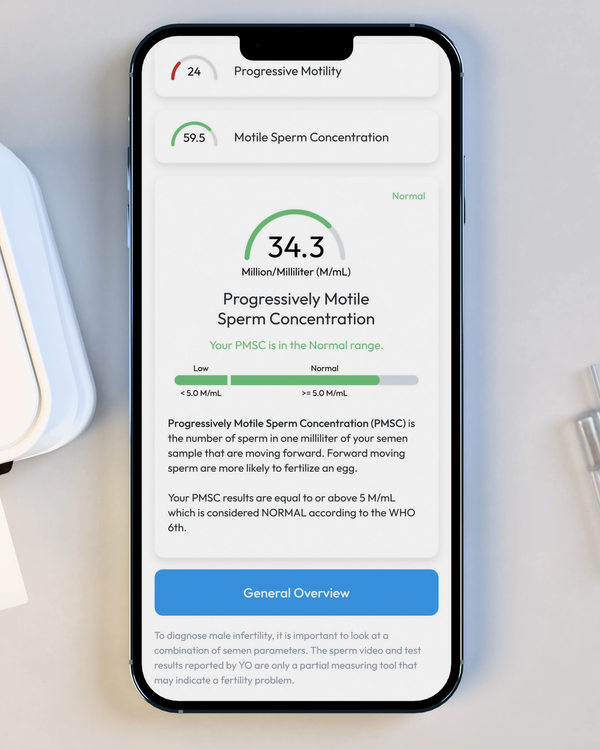
Progressive Motile Sperm Concentration, M/mL
PMSC, or Progressive Motile Sperm Concentration, represents the number or forward-moving sperm in your sample, per ML.

YO is Future Proof
The World Health Organization (WHO) publishes comprehensive guidelines for male fertility testing and treatment. Medical Electronic Systems (MES) and YO Home Sperm Test adhere to the latest WHO standards, as outlined in the current 6th Edition Manual.
As an industry leader in the fertility space, MES is dedicated to meeting not only current WHO standards, but also all FDA, ASRM, AUA, CLIA, CAP, and international regulatory standards to provide a world-class solution for couples and men worldwide.
The Importance of These Results
Your fertility journey deserves clarity and confidence. By measuring sperm concentration, motility, and progressive motility, you gain essential insights into his reproductive health. These key results, following WHO 6th Edition guidelines, empower you to plan your next steps with intelligence and confidence, ensuring you make informed decisions for your future.
Monitoring Reproductive Health in Private
Our achievements in numbers
Expert Opinions
What Medical Professionals Say About YO